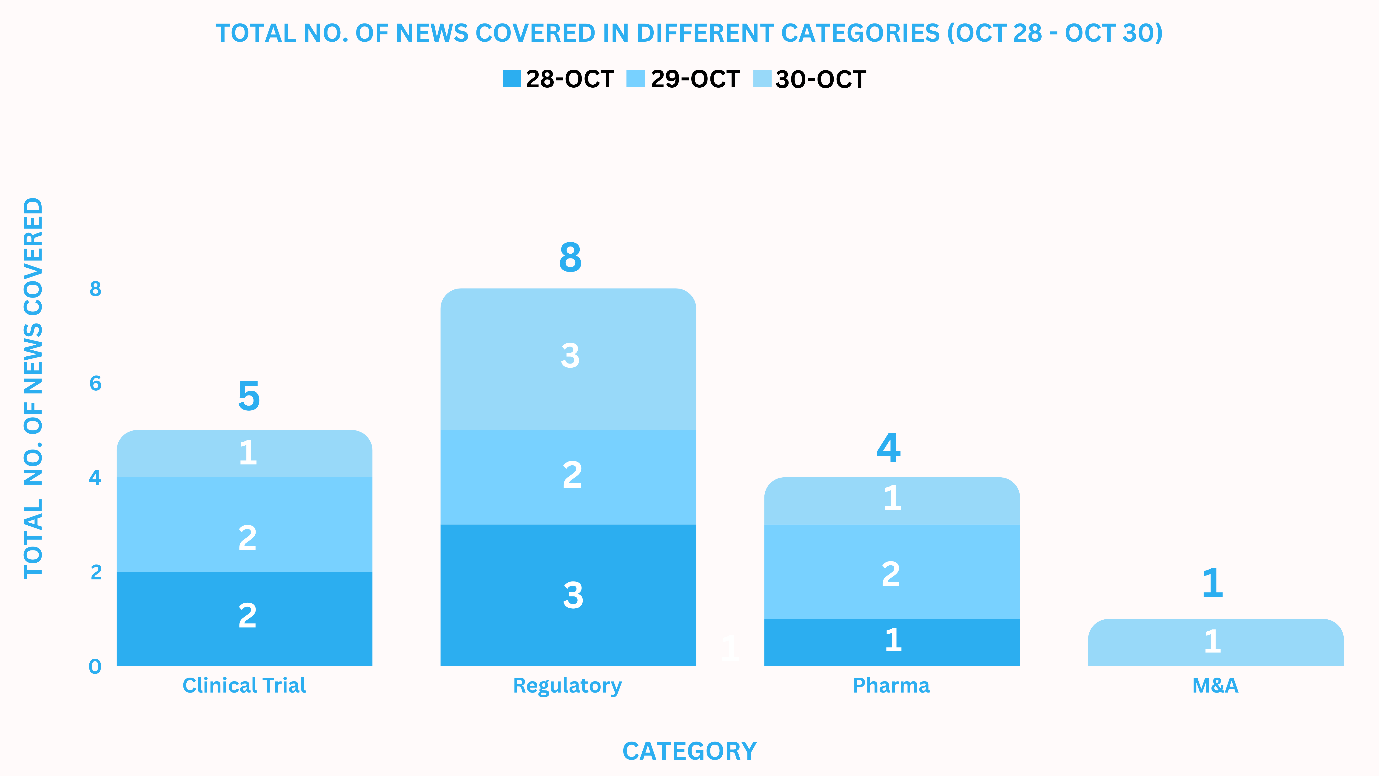
PharmaShots Weekly Snapshots (October 28 – October 30, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Regulatory & M&A. Check out our full report below:

Biogen Reports Positive Data of Felzartamab to Treat IgA Nephropathy (IgAN) at ASN Kidney Week 2024
Read More: Biogen
Novartis Reports Data from P-III (APPEAR-C3G) Study of Fabhalta (Iptacopan) in C3 Glomerulopathy (C3G)
Read More: Novartis
Shuttle Pharma Reports the Completion of Clinical Trial Site Enrollment for the P-II Study of Ropidoxuridine for Glioblastoma
Read More: Shuttle Pharma
EyePoint Pharmaceuticals Reports Data From Ongoing P-II (VERONA) Study of Duravyu in Diabetic Macular Edema (DME)
Read More: EyePoint Pharmaceuticals
Rallybio Receives the CTAs’ Approval for P-II Study of RLYB212 to Treat HPA-1a Alloimmunization and FNAIT
Read More: Rallybio

Modalis Therapeutics’ MDL-101 Gains the US FDA’s Orphan Drug Designation to Treat Congenital Muscular Dystrophy Type 1A (LAMA2-CMD)
Read More: Modalis Therapeutics
European Patent Office (EPO) Grants Approval to Biogen’s Tecfidera Patent
Read More: Biogen
Iterum Therapeutics’ Orlynvah (Oral Sulopenem) Receives the US FDA’s Approval to Treat uUTIs
Read More: Iterum Therapeutics
Kind Pharmaceutical’s AND017 Gains the US FDA’s Orphan Drug Designation to Treat SCD
Read More: Kind Pharmaceutical
AstraZeneca’s Fasenra Receives EU Approval for Eosinophilic Granulomatosis with Polyangiitis
Read More: AstraZeneca
The US FDA Approves Novartis’ Scemblix for Newly Diagnosed Ph+ CML in Chronic Phase
Read More: Novartis
Shorla Oncology Reports the US FDA’s Expanded Approval of Jylamvo for Pediatric Indications
Read More: Shorla Oncology
Johnson & Johnson’s Edurant Receives the EC Approval for Expanded Use as HIV-1 Therapy in Pediatric Individuals
Read More: Johnson & Johnson

Astellas Reports MAA Withdrawn for Avacincaptad Pegol (ACP) in the EU
Read More: Astellas
Monte Rosa Therapeutics and Novartis Collaborate to Advance VAV1-directed Molecular Glue Degraders (MGD)
Read More: Monte Rosa Therapeutics & Novartis
Lisata Therapeutics and the University of Cincinnati Enters into Sponsored Agreement for Certepetide to Treat Endometriosis
Read More: Lisata Therapeutics and the University of Cincinnati
Biogen and Neomorph Collaborate to Discover and Develop Molecular Glue Degraders (MGD)
Read More: Biogen and Neomorph

GSK to Acquire Chimagen Biosciences’ CMG1A46, Expanding its Immunology Pipeline
Read More: GSK and Chimagen Biosciences
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com